Our Research
A striking aspect of developmental processes is that organs and organisms grow over a long time and drastically increase their mass. Yet, despite the complexity of organogenesis, final organ size is precisely controlled. This is evident, for example, when observing the nearly identical size of bilateral organs, or the highly coordinated growth among body parts that ensures proper body proportions. A challenge in developmental biology is, therefore, to understand how organs increase their mass, measure their size, and stop growing appropriately. Our lab is leveraging recent advances in genetics, microscopy, image analysis, and sequencing to bring new insights into this fundamental question.
In multicellular organisms, growth is controlled at different scales (organism, organ/tissue, cell, organelles/intracellular structures) to establish species-specific organ shapes and proportions. We are using Drosophila melanogaster to investigate how different levels of control are integrated to determine the final size of organs and organisms. An important aspect of our research is to understand the interplay between organ-intrinsic genetic programs and systemic factors, which relay information about the environment and mediate the crosstalk between individual organs and organismal physiology.
To tackle the question of size determination during development, we develop two complementary approaches:
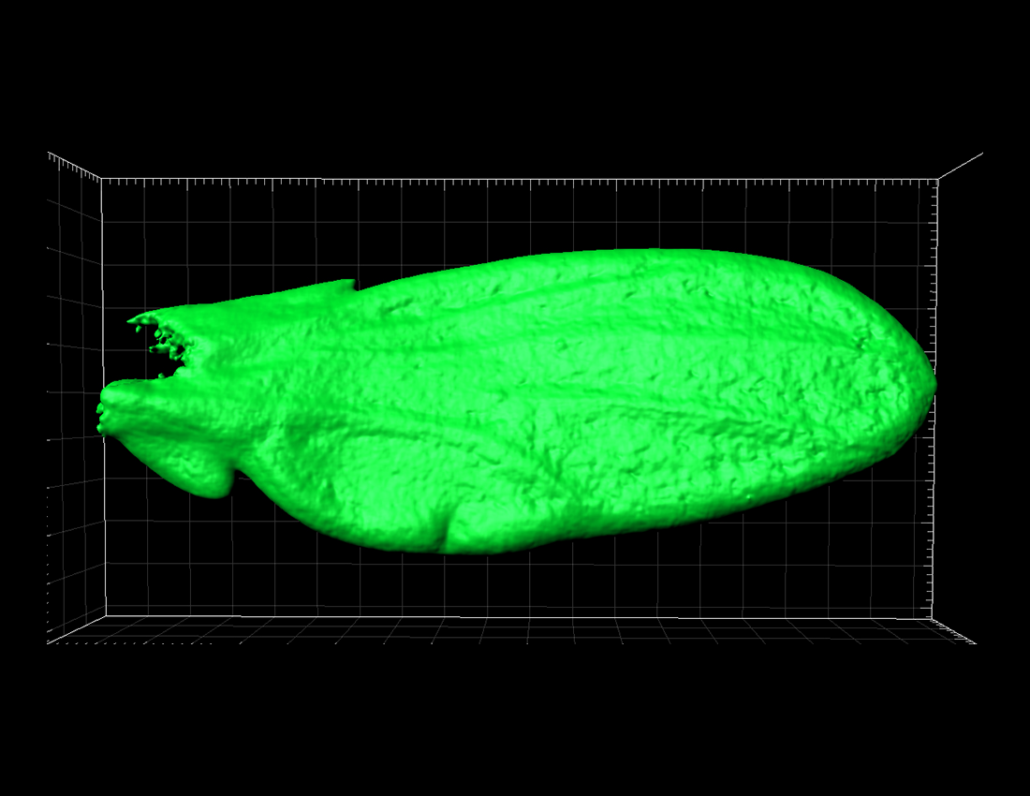
- Investigating the mechanisms controlling organ size in physiological conditions. Which signals and cellular behaviors drive tissue growth? How do organs stop growing? How is extrinsic information integrated to adjust the target size of an individual organ with overall body size? Is growth homogeneous within a tissue? How is growth coupled with morphogenesis? Our aim is to build on the well-established model of the Drosophila wing primordium to address these questions and establish a detailed and comprehensive view of size control in this system. To do so, we are implementing state-of-the-art techniques to image growing tissues, measure growth parameters, and perturb gene expression.
- Characterizing the responses to growth perturbations. Growth control also relies on the plasticity of growth-regulatory mechanisms to ensure the robustness of adult phenotypes. One way to investigate the key mechanisms for final size determination is to perturb the system and study how it recovers. How do organs detect a growth perturbation? Which compensatory mechanisms are at play to ensure that the appropriate target size is attained? Do organs measure their size, and if so, how? How is developmental precision achieved? We are taking advantage of the genetic toolkit in Drosophila to precisely modulate gene expression in time and space, allowing us to induce controlled growth perturbations of different natures. This approach aims to uncover some of the mechanistic logic underlying size control and has potential implications for studying growth-related disorders.
Read more
- The lab is officially opening on July 1st, 2024!
- We have an open post-doc position! Passionate about developmental biology, Drosophila genetics, microscopy or transcriptomics? Don’t hesitate to contact us (laura.boulan@crbm.cnrs.fr) if you’d like to take part in this new adventure! Starting date: nov-dec 2024.
Funding
ATIP-Avenir Programme
Université de Montpellier
Publications
2025
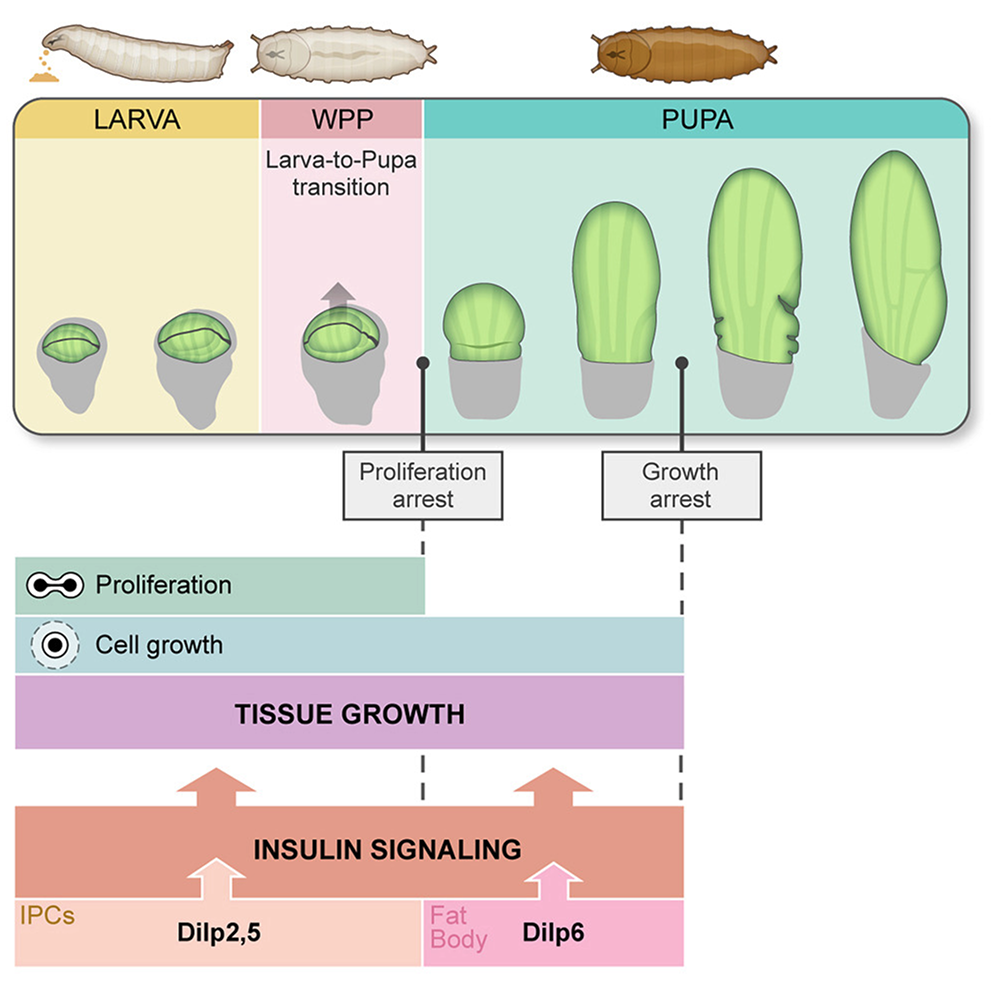
- A switch to non-proliferative growth sustains Drosophila wing development during the early pupal stage. El Marzkioui K, Gaugué I, De Giorgio E, Anguille C, Léopold P, Boulan L. Curr Biol. 2025 Aug 18;35(16):4043-4049.e3. Pubmed
2022
- A Dilp8-dependent time window ensures tissue size adjustment in Drosophila. Blanco-Obregon D, El Marzkioui K, Brutscher F, Kapoor V, Valzania L, Andersen DS, Colombani J, Narasimha S, McCusker D, Léopold P, Boulan L.Nat Commun. 2022 Sep 26;13(1):5629.. Pubmed
2021
- What determines organ size during development and regeneration? Boulan L, Léopold P. Development. 2021 Jan 11;148(1):dev196063. Pubmed
2019
- Quantitative FLIM-FRET Microscopy to Monitor Nanoscale Chromatin Compaction In Vivo Reveals Structural Roles of Condensin Complexes. Llères D, Bailly AP, Perrin A, Norman DG, Xirodimas DP, Feil R. Cell Rep. 18:1791-1803. Pubmed
2015
- Drosophila Lgr3 Couples Organ Growth with Maturation and Ensures Developmental Stability. Colombani J, Andersen DS, Boulan L, Boone E, Romero N, Virolle V, Texada M, Léopold P. Curr Biol. 2015 Oct 19;25(20):2723-9. Pubmed
- The Systemic Control of Growth. Boulan L, Milán M, Léopold P.Cold Spring Harb Perspect Biol. 2015 Aug 10;7(12):a019117.. Pubmed
2014
- Neuronal glycogen synthesis contributes to physiological aging. Sinadinos C, Valles-Ortega J, Boulan L, Solsona E, Tevy MF, Marquez M, Duran J, Lopez-Iglesias C, Calbó J, Blasco E, Pumarola M, Milán M, Guinovart JJ.Aging Cell. 2014 Oct;13(5):935-45. Pubmed
- Mei-P26 mediates tissue-specific responses to the Brat tumor suppressor and the dMyc proto-oncogene in Drosophila. Ferreira A, Boulan L, Perez L, Milán M. Genetics. 2014 Sep;198(1):249-58. Pubmed
2013
- bantam miRNA promotes systemic growth by connecting insulin signaling and ecdysone production. Boulan L, Martín D, Milán M. Curr Biol. 2013 Mar 18;23(6):473-8 Pubmed
Growth control during development
Team Members
(AI-Recherche) +33 (0)4 34 35 95 13 |
|
(Chercheur CRCN) +33 (0)4 34 35 95 13 |
|
(Chercheur) +33 (0)4 34 35 95 13 |
|
(Stagiaire) +33 (0)4 34 35 9719 |
Contact us
Replace the name and address below with that of the member to contact
Firstname.name@crbm.cnrs.fr