Our Research
Tubular epithelial organization is a common feature of many developing tissues, including kidneys. Its disruption in pathologies, such as polycystic kidney disease and cancer, highlights the importance of understanding the coordination of all dynamic cellular processes involved in its formation. Polycystic kidney disease is characterized by kidney tubules and lumen disorganization and has long been associated exclusively with dysfunctions of cilia, sensory organelles required for proper cellular functions within tissues. However, recent results, including ours, indicate that intracellular transport complexes involved in kidney cyst formation and initially described for their ciliary roles, also have non-ciliary functions that may contribute to the disease. Our overall objective is to study the non-ciliary roles of these transport complexes, primarily during cell division, in order to identify the cellular mechanisms that contribute to kidney tube morphogenesis in normal and pathological conditions. Our work should lead to an integrated view of the cellular mechanisms involved in kidney tubule morphogenesis and should bring insights into their contributions to pathologies such as kidney cyst formation and cancer.
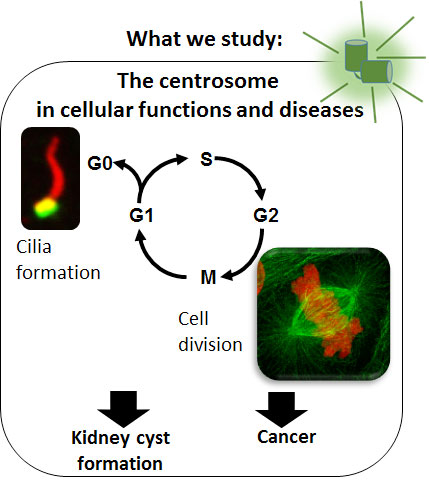
Centrosomes are microtubule-organizing centers that function at multiple stages of the cell cycle. In non-cycling cells, they contribute to the formation of cilia. In dividing cells, centrosomes are required for mitotic spindle organization and orientation. Centrosome defects have been associated with many diseases, such as ciliopathies, polycystic kidney disease and cancer.
The IntraFlagellar Transport machinery (IFT) in cellular functions and diseases. Cilia proteins of the IFT machinery were initially identified for their link to polycystic kidney disease and their role in cilia. Even if polycystic kidney disease has long been associated exclusively with cilia dysfunctions, recent findings, including our own, suggest that IFT proteins also have non-ciliary roles that could contribute to this pathology. We propose to study the contribution of cell division to the formation of renal tubules in normal and pathological conditions, by focusing on the following questions: (1) What are the non-ciliary roles of IFT proteins? (2) How is cell division organized during kidney tubes morphogenesis? (3) What is the contribution of cell division defects to kidney cyst formation? (4) What is the contribution of IFT proteins to cancer cell division, proliferation and survival?
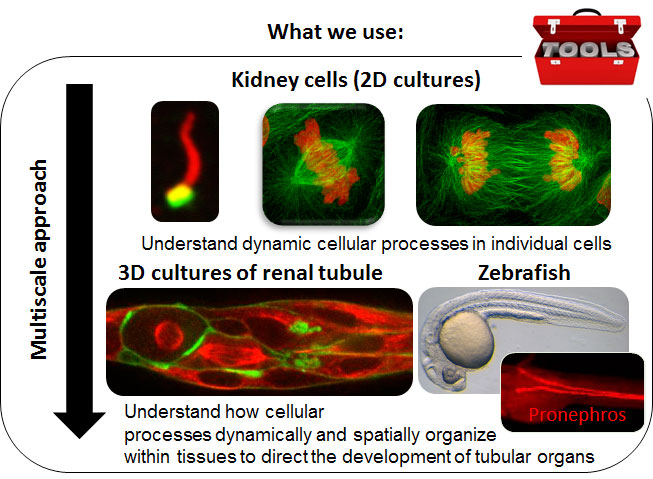
Proposed approaches to link the cellular scale to the tissue scale. To address questions at the crossroad between cellular biology and diseases, we use cutting edge microscopy techniques on two-dimensional (2D) and three-dimensional (3D) cultures of kidney cells. Indeed, 3D epithelial culture systems, which allow epithelial cells to organize into structures that resemble their in vivo architecture, have emerged as excellent models to study epithelial morphogenesis in a biologically relevant context. To validate and broaden our results, we also take advantage of zebrafish, a powerful in vivo model for visualizing sub-cellular processes during kidney tubule morphogenesis and an attractive vertebrate model to study polycystic kidney disease and cancer.
Read more
The group leader
Since 2012
Group leader- CRBM, Montpellier, France
Cilia Centrosome and pathologies group
CR1 CNRS, ANR CHAIRE D’EXCELLENCE 2012
09/2005-01/2012
Postdoctoral Research Associate, UMASS Medical School, Worcester MA, USA
Laboratory: S. Doxsey – Molecular Medicine
Collaboration: N. Lawson (Zebrafish lab, PGFE, UMASS Medical School)
Projects: The centrosome in cell cycle regulation /Centrosome defects in ciliopathy
2001 – 2005
PhD Research, Marseille Cancer Institute, INSERM U599, Marseille, France
Laboratory: D. Birnbaum – Molecular Oncology
PhD research project : Centrosomal abnormalities and oncogenesis
2000 – 2001
Master Research Marseille Cancer Institute, INSERM U599 Marseille France
Laboratory: J. Imbert. Collaboration P. Naquet
Project: Molecular basis of the immune dysfunctions associated with type 1 diabetes
03/ 2001
Student research Marseille Cancer Institute, INSERM U599, Marseille, France
Laboratory: D. Olive – Tumor Immunology
Project: Dendritic cells and leukemias
07- 08/ 1999
Student research Center of Immunology of Marseille, France
Supervisors: C. Nguyen/ B. Jordan
Technologies Avancées en Génomique et Clinique
Project: RNA extraction- Microarray technology
07-08/ 1997
NEOCRIN Company, Diabetes, Irvine, CA, USA
Cellular transplant for the treatment of insulin-dependent diabetes mellitus
Prices and awards
- 2013 Fondation Schlumberger pour l’Education et la Recherche (FSER) laureate.
- 2011 Young Researcher Prize of the SBCF (Société de Biologie Cellulaire de France ) has been be awarded to Bénédicte Delaval on Friday, March 30th, 2012 at the CRBM.
The award ceremony and Bénédicte’s presentation can be seen on http://www.webtv.univ-montp2.fr/11567/conference-crbm/
Funding
ANR LUCELL (Bénédicte Delaval)
Publications
2025
- An Auxin Inducible Degradation System to Study Mklp2 Functions in MDCK Epithelial Cells. Rodriguez M, Simon V, Delaval B, Vitre B. Biol Cell. 2025 Jun;117(6):e70015. Pubmed
2022
- The emergence of spontaneous coordinated epithelial rotation on cylindrical curved surfaces. Glentis A, Blanch-Mercader C, Balasubramaniam L, Saw TB, d’Alessandro J, Janel S, Douanier A, Delaval B, Lafont F, Lim CT, Delacour D, Prost J, Xi W, Ladoux B. Sci Adv. 2022 Sep 16;8(37):eabn5406.. Pubmed
2020
- Editorial: Dissecting the Intraflagellar Transport System in Physiology and Disease: Cilia-Related and -Unrelated Roles. Finetti F, Pan J, Qin H, Delaval B. Front Cell Dev Biol. 8:615588 Pubmed
- Non-ciliary Roles of IFT Proteins in Cell Division and Polycystic Kidney Diseases. Vitre B, Guesdon A, Delaval B. Front Cell Dev Biol. 2020 Sep 22;8:578239 Pubmed
- Intraflagellar Transport Complex B Proteins Regulate the Hippo Effector Yap1 during Cardiogenesis. Peralta M, Ortiz Lopez L, Jerabkova K, Lucchesi T, Vitre B, Han D, Guillemot L, Dingare C, Sumara I, Mercader N, Lecaudey V, Delaval B, Meilhac SM, Vermot J. Cell Rep. 32(3):107932 Pubmed
- mTOR and S6K1 Drive Polycystic Kidney by the Control of Afadin-dependent Oriented Cell Division. Bonucci M, Kuperwasser N, Barbe S, Koka V, de Villeneuve D, Zhang C, Srivastava N, Jia X, Stokes MP, Bienaimé F, Verkarre V, Lopez JB, Jaulin F, Pontoglio M, Terzi F, Delaval B, Piel M, Pende M. Nat Commun ;11(1):3200 Pubmed
- IFT proteins interact with HSET to promote supernumerary centrosome clustering in mitosis. Vitre B, Taulet N, Guesdon A, Douanier A, Dosdane A, Cisneros M, Maurin J, Hettinger S, Anguille C, Taschner M, Lorentzen E, Delaval B. EMBO Rep. e49234 Pubmed
2019
- IFT88 controls NuMA enrichment at k-fibers minus-ends to facilitate their re-anchoring into mitotic spindles. Taulet N, Douanier A, Vitre B, Anguille C, Maurin J, Dromard Y, Georget V, Delaval B. Sci Rep. 9(1):10311. Pubmed
2017
- IFT proteins spatially control the geometry of cleavage furrow ingression and lumen positioning. Taulet N, Vitre B, Anguille C, Douanier A, Rocancourt M, Taschner M, Lorentzen E, Echard A, Delaval B. Nat Commun. 8(1):1928. Pubmed
- Flotillins control zebrafish epiboly through their role in cadherin-mediated cell-cell adhesion. Morris EA, Bodin S, Delaval B, Comunale F, Georget V, Costa ML, Lutfalla G, Gauthier-Rouvière C. Biol Cell. Pubmed
2016
- The use of the NEDD8 inhibitor MLN4924 (Pevonedistat) in a cyclotherapy approach to protect wild-type p53 cells from MLN4924 induced toxicity. Malhab LJ, Descamps S, Delaval B, Xirodimas DP. Sci Rep. 6:37775. Pubmed
2015
- New frontiers: discovering cilia-independent functions of cilia proteins. Vertii A, Bright A, Delaval B, Hehnly H, Doxsey S. EMBO Rep. 16:1275-87. Pubmed
2014
- Non-ciliary functions of cilia proteins. Taulet N, Delaval B. Med Sci (Paris). 30:1040-6. Pubmed
- Identification of a mitotic Rac-GEF, Trio, that counteracts MgcRacGAP function during cytokinesis. Cannet A, Schmidt S, Delaval B, Debant A. Mol Biol Cell. 25:4063-71. Pubmed
2011
- The cilia protein IFT88 is required for spindle orientation in mitosis. Delaval B, Bright A, Lawson ND, Doxsey S. Nat Cell Biol. 13(4):461-8. Pubmed
- Centrin depletion causes cyst formation and other ciliopathy-related phenotypes in zebrafish. Delaval B, Covassin L, Lawson ND, Doxsey S. Cell Cycle. 10(22):3964-72. Pubmed
2010
- Pericentrin in cellular function and disease. Delaval B, Doxsey SJ. J Cell Biol. 188(2):181-90. Pubmed
2009
- Distinct roles of BARD1 isoforms in mitosis: full-length BARD1 mediates Aurora B degradation, cancer-associated BARD1beta scaffolds Aurora B and BRCA2. Ryser S, Dizin E, Jefford CE, Delaval B, Gagos S, Christodoulidou A, Krause KH, Birnbaum D, Irminger-Finger I. Cancer Res. 69(3):1125-34. Pubmed
2008
- Dwarfism, where pericentrin gains stature. Delaval B, Doxsey S. Science. 2008 Feb 8;319(5864):732-3. Pubmed
Centrosome, cilia and pathologies

Bénédicte DELAVAL
Group leader (Research Director CNRS)
Team Members
(IE-Recherche) +33 (0)4 34 35 95 03 |
|
(CRCN) +33 (0)4 34 35 95 30 |
|
(Chercheur DR2) +33 (0)4 34 35 95 30 |
|
(CRCN) +33 (0)4 34 35 95 31 |
|
(CRCN) +33 (0)4 34 35 95 31 |
|
(Maître de conférences) +33 (0)4 34 35 95 37 |
|
(IR-Recherche) +33 (0)4 34 35 95 31 |
|
(Doctorant) +33 (0)4 34 35 95 37 |
|
(Stagiaire) +33 (0)4 34 35 95 31 |
The team is affiliated to the French Society for Cell Biology (SBCF) and B. Delaval is an elected member of the board of the SBCF.

SBCF Young Research Award, 10 years later …
see the video (in french)
Contact us
Replace the name and address below with that of the member to contact
firstname.name@crbm.cnrs.fr
Highlight April 2020

![]()