Our Research
Gastrulation as model to understand tissue plasticity and invasiveness
Animal and human cells have the remarkable capacity to adhere to each other and to actively migrate. These two fundamental properties are at the core of morphogenesis, responsible to build tissues and organs during embryonic development. They are similarly crucial for homeostasis of adult tissues (see permanent renewal of our digestive track and skin), and for wound healing and tissue regeneration. They are also central to the process of cancer metastasis.
How can cells move while tightly holding together?
The central and still largely unsolved question is how cells migrate within a tissue, and how this motility is regulated to provide the tissue with the right dynamic properties. This involves two major processes: cell motility, to rapidly change shape and form dynamic protrusions, and remodelling of cell-cell adhesions, to detach from one neighbour and reattach to another. It turns out that regulation of the actomyosin cytoskeleton is central to both processes.
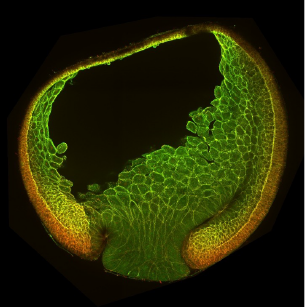
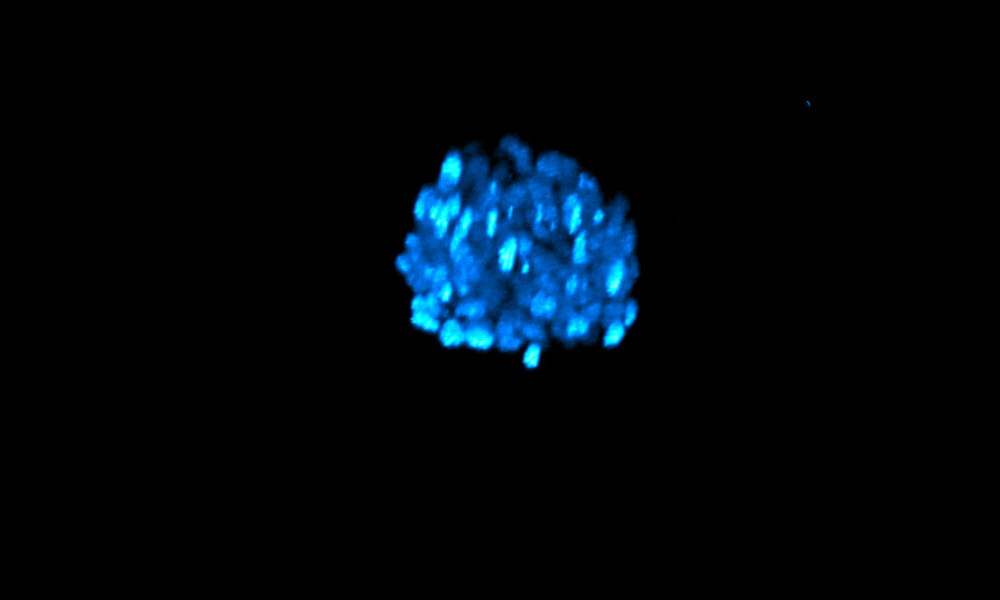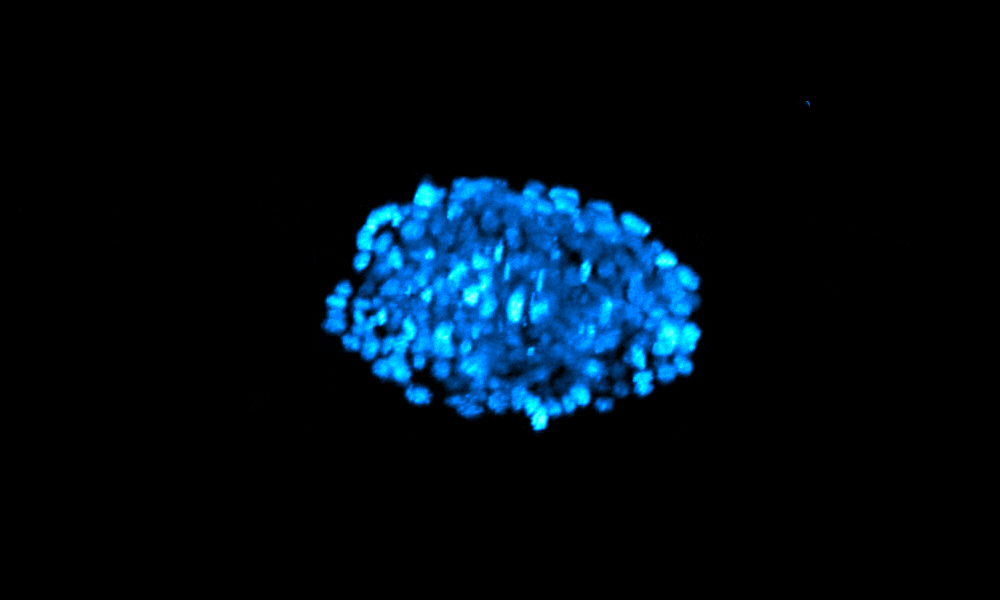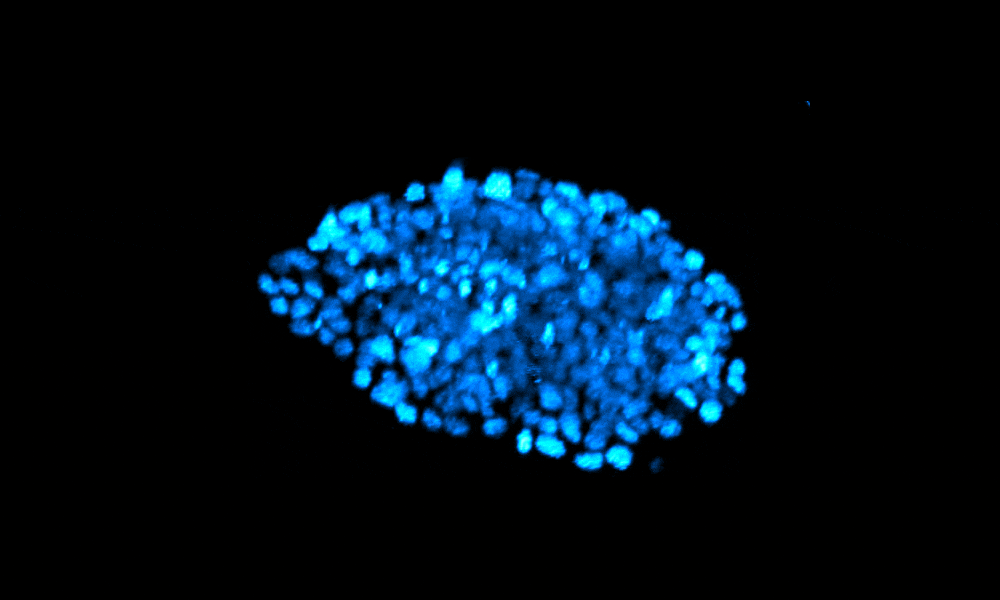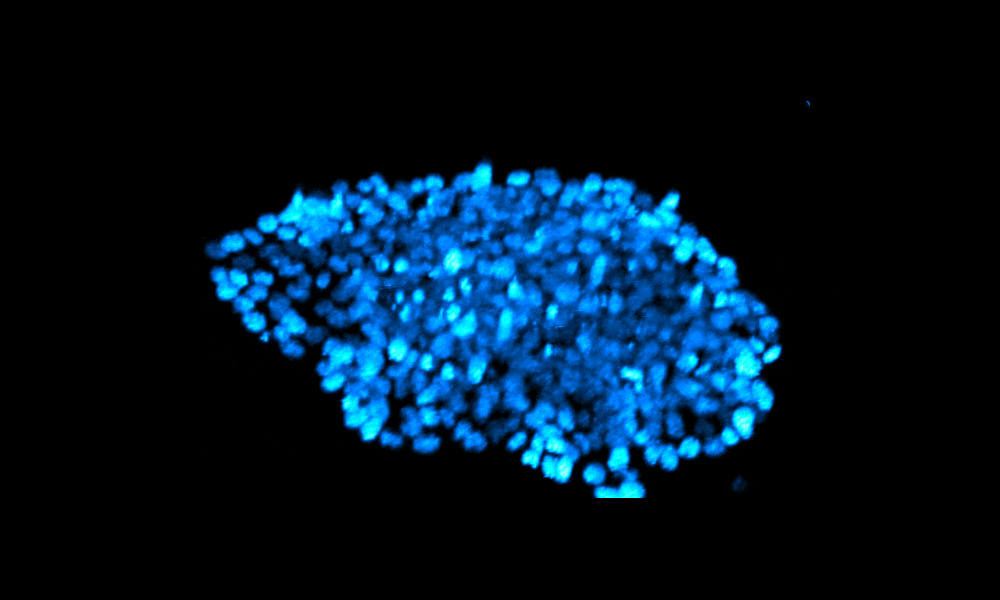
The Xenopus gastrula: Whole mount immunostaining of a late gastrula. Most green cells have migrated inside. Credit: Christine Fagotto-Kaufmann.
A cellular approach to embryonic development.
Gastrulation is a fundamental step of embryogenesis, and a powerful model to study tissue plasticity. Mesoderm and endoderm tissues massively migrate to invade the interior of the embryo. These movements are fast, stereotypical and coordinated, thus giving predictable and exquisitely sensitive phenotypes. Xenopus embryos are ideally suited to manipulate any molecular process through loss- and gain-of-function, in combination with expression of a variety of molecular markers for live imaging. Most importantly, they offer the unique possibility to dissect specific regions for in vitro study of tissues (“gastruloids”) and isolated cells. This allows to use the whole panel of Cell Biology techniques to explore the cellular basis of tissue plasticity in an embryonic, thus physiologically highly relevant, system.
Biophysics, modelling and simulation.
We have also implemented various biophysical approaches, such as pipette aspiration, force inference and traction force microscopy, and we have established close collaborations with physicists to implement modelling and simulation.
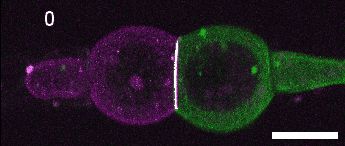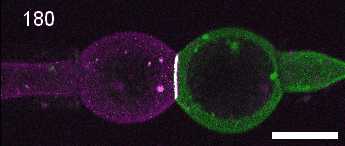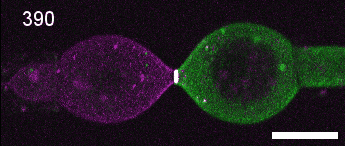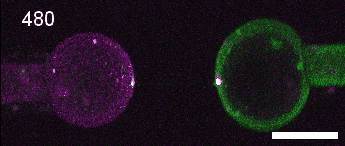
A close look at cell-cell contact remodelling. Two mesoderm cells expressing cadherin in two colours are held using micropipettes, brought together to form an adhesion interface, then pulled away to analyse cadherin dynamics as the contact is disassembled. Credit: David Rozema.
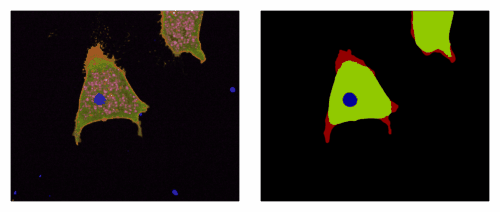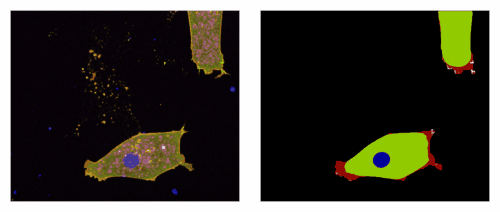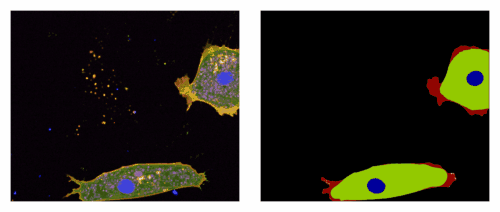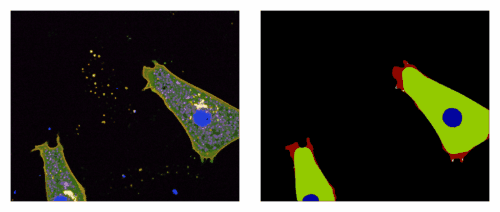
Morphogenetic properties of human cancer cells
Cancer cells are known to reuse embryonic processes. Consistently, the molecules and mechanisms that we uncover in gastrula embryos also participate to cancer development. We thus also use in parallel cell lines to validate our findings for in the context of collective behaviour of cancer cells (see our work on the tumour-associated protein EpCAM).
Collective migration of a spheroid of a breast carcinoma spheroid. Nuclei labelled with SiR-DNA. Credit: Azam Aslemarz
Recent major findings:
1) Make a tissue soft to make it dynamic: Kashkooli et al, Plos Biology 2021. While for epithelia one generally considers that movements are powered by increasing tension, we find that in the mesoderm, a prototypic mesenchymal tissue, it’s the other way around, its movement is triggered by reducing myosin-based tension.
2) Cadherin peeling: Rozema et al, Dev Cell 2025 in press. Mesoderm cells dismantle their cell-cell contacts by a smooth peeling process, with freed cadherins diffusing away along the plasma membrane.
3) An EpCAM/Trop2 mechanostat: Azam et al, EMBO J 2025. EpCAM and Trop2 are sister proteins, both highly expressed in human carcinoma. We show that they play a subtle game on myosin regulation to control collective migration of carcinoma cells.
Read more
Pr François FAGOTTO
BIOGRAPHY
Of mixed Swiss French and Venetian background, I studied Biology at the University of Neuchâtel, one of the smallest universities in Europe. I stayed there for my PhD, choosing a not-so-common topic, i.e. yolk degradation (embryonic storage) in an exotic model, an African tick. In 1991, I went for a first postdoc at Columbia University, New York City, in the lab of Fred Maxfield, a pioneer of live imaging and of the study of endosomes. There I still fumbled with yolk “late endosomes” and regulation of their pH.
Leaving yolk aside for good, I then crossed Manhattan from the West to the East Side to join the lab of Barry Gumbiner at the Sloan-Kettering Institute, right at the time where the dual role of β-catenin in adhesion and Wnt signalling was being discovered. I explored various facets of this topic, from its role during embryonic development to the mechanism of β-catenin nuclear transport. Toward the end of this second postdoc, in a collaboration with the lab of Frank Constantini, I discovered Axin, the scaffold protein building the β-catenin degradation complex.
In 1997, I started my own team as 5-year junior group leader at the Max-Planck Institute of Developmental Biology in Tübingen, continuing to work on β-catenin and Axin. In 2002, I moved to McGill University, Montreal, where I held a Canada Research Chair. There I fully entered the field of morphogenesis, using the Xenopus gastrula as model to decipher the cellular mechanisms responsible for cell sorting and separation of embryonic tissues.
Pressed by my longing for centennial stone buildings and for alpine pastures, I came back to old Europe in 2015, as Professor at the University of Montpellier and team leader at the CRBM-CNRS Institute. This last move coincided with starting to explore a new aspect of morphogenesis, tackling the basis of tissue motility and remodelling using a combination of cellular and biophysical approaches.
CV and complete list of publications can be found at https://orcid.org/0000-0002-1495-2112
MAJOR RESEARCH CONTRIBUTIONS
Separate functions of β-catenin in Wnt signaling and cell adhesion
Funayama et al, J. Cell Biol. 1996
β-catenin had just been discovered as central cadherin interactor for cell adhesion, but also as component of the Wnt signalling pathway. We provided the first demonstration that β-catenin localized in the nucleus upon Wnt activation, that signalling and adhesive functions could be dissociated, and that cadherin binding sequestered β-catenin, thus antagonizing Wnt signalling.
Discovery of Axin, the central scaffold protein of the Wnt pathway
Zeng*, Fagotto*, et al. Cell 1997 (* First equal authors)
Despite the knowledge of several components of the pathway, the mechanism regulating b-catenin had remained quite puzzling. Axin turned out to be a key molecule: it functions as a scaffold protein that can recruit basically all cytoplasmic components of the pathway. Its discovery provided for the first time a coherent view of the pathway, and gave a strong impulse to the field, allowing the identification of many new interacting partners, and of cross talks with other pathways.
β-catenin nuclear transport
Fagotto et al, Curr. Biol. 1998; Wiechens and Fagotto, Curr. Biol. 2001
We showed that β-catenin can freely shuttle in and out of the nucleus in a very unconventional way, independently of the classical nuclear transport pathways. β-catenin may actually have evolved from an ancestor “importin”, and whether it may have retained a function as nuclear transport receptor is an open question (Fagotto, EMBO Rep. 2013).
The Signaling Atlas of Xenopus Development
Schohl and Fagotto, Development 2002
In this study, we produced an 4D atlas of four major pathways involved in embryonic development, from early blastula stage to hatching. This atlas provided the first global view of the complex sequence of signals involved in the development of an animal organism. It is recognized as a major must-read resource by the Xenopus community.
Embryonic boundaries rely on local cell repulsion
Rohani et al, PLoS Biology 2011
This study provided the first high resolution images of cell behavior at an embryonic boundary. It revealed that ectoderm and mesoderm of the Xenopus gastrula remained separated due to constant cycles of adhesion-deadhesion controlled by ephrin-Eph signaling across the boundary. This work overturned the prevailing models, which postulated that tissue separation relied on global adhesive or contractile differences between the tissues.
Tumor associated EpCAM is a PKC inhibitor that controls cell adhesion and motility.
Maghzal et al, Developmental Cell 2013
EpCAM is a major marker for carcinoma. While it was long thought to function as cell adhesion molecule, we had found that in Xenopus embryos it stimulates cell adhesion and cell migration indirectly by inhibiting PKC signaling (Maghzal et al, J. Cell Biol. 2010). We reported here that EpCAM cytoplasmic tail contains a short peptide segment that directly binds and inhibits intracellular kinases, called novel PKCs. Other proteins involved in adhesion or repulsion also carry a similar PKC-inhibitory domain. We thus uncovered a novel mechanism of intracellular signaling regulation by cell membrane proteins.
Regulation of cadherin adhesion along the embryonic boundary
Fagotto et al, Developmental Cell 2013
Using formation of the notochord in Xenopus, we showed that repulsive cues generate acute actomyosin contractility at the tissue interface, locally inhibiting cadherin clustering, thus explaining the low adhesion across the boundary and high efficiency of cell sorting. This is the first in vivo evidence for regulation of cadherin clustering, a mechanism which may have a broad impact on cell-cell adhesion and morphogenesis.
A “tissue identity code” made of selective ephrin-Eph pairs
Rohani et al, PLoS Biology 2014
We demonstrated that vertebrate embryonic cells recognize self from non-self, thus restricting repulsion at tissue boundaries, through a combination of multiple ephrins and Eph receptors, simply based on binding selectivity and asymmetric expression. This study, dissecting ephrin-Eph networks at three different Xenopus boundaries, provides a molecular mechanism for the “cell affinities” postulated more than seventy years ago to explain how different cell types can sort into separate tissues.
High heterotypic interfacial tension as mechanistic basis of embryonic boundaries.
Canty et al, Nature Comm 2017
Formal, definitive demonstration through experimental data and biophysical modelling that cell sorting and tissue separation cannot be generated by global differences in cell adhesion or contractility, as widely previously assumed, but are instead driven by local high local tension at the interface between the cell types. For reviews on this fundamental concept, see Fagotto, Development 2014, and Fagotto, Sem. Cell Dev. Biol. 2020.
Ectoderm to mesoderm transition by downregulation of actomyosin contractility
Kashkooli, Rozema et al, PLoS BIOLOGY 2021
Discovery that mesoderm migration during gastrulation is triggered by a developmentally-controlled inhibition of the Rho-Rock-myosin pathway, and identification of corresponding tissue-specific Rho inhibitors. This view of collective migration of a mesenchymal-like tissue stimulated by cell “softening” contrasts with the classical model for epithelial morphogenesis, which considers high contractility as the motor of tissue remodelling.
Funding
ANR Inter-s-cal (François Fagotto)
Publications
2025
2024
2023
2022
-
-
An EpCAM/Trop2 mechanostat differentially regulates individual and collective migration of human carcinoma cells.Pubmed
2021
- Ectoderm to mesoderm transition by down-regulation of actomyosin contractility. Kashkooli L, Rozema D, Espejo-Ramirez L, Lasko P, Fagotto F. PLoS Biol. 2021 Jan 6;19(1):e3001060 Pubmed
- EpCAM cellular functions in adhesion and migration, and potential impact on invasion: A critical review. François Fagotto , Azam Aslemarz. Biochim Biophys Acta Rev Cancer, 2020 Dec;1874(2):188436. Pubmed
- Tissue segregation in the early vertebrate embryo. François Fagotto. Semin Cell Dev Biol. 2020 Nov;107:130-146. Pubmed
- Cell sorting at embryonic boundaries. François Fagotto. Semin in Cell Dev Biol. 2020 Nov;107:126-129. Pubmed
- EpCAM as Modulator of Tissue Plasticity. François Fagotto. Cells; 2020 Sep 19;9(9):2128. Pubmed
2018
- Limited significance of the in situ proximity ligation assay. Azam Alsemarz, Paul Lasko, François Fagotto. bioRxiv 411355 (November 05, 2018) Pubmed
2017
- Sorting at embryonic boundaries requires high heterotypic interfacial tension. Canty L, Zarour E, Kashkooli L, François P, Fagotto F. Nat Commun. 2017 Jul 31;8(1):157. Pubmed
2015
- Regulation of cell adhesion and cell sorting at embryonic boundaries. Fagotto F. Curr Top Dev Biol. 2015;112:19-64. Pubmed
- Nuclear β-Catenin under Control. Fagotto F. Dev Cell. 2015 Jun 22;33(6):625-6. Pubmed
2014
- Regulation of the phosphorylation and nuclear import and export of β-catenin by APC and its cancer-related truncated form. Wang L, Liu X, Gusev E, Wang C, Fagotto F. J Cell Sci. 2014 Apr 15;127(Pt 8):1647-59. Pubmed
- Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. Rohani N, Parmeggiani A, Winklbauer R, Fagotto F. PLoS Biol. 2014 Sep 23;12(9):e1001955. Pubmed
- The cellular basis of tissue separation. Fagotto F. Development. 2014 Sep;141(17):3303-18. Pubmed
- Ephrin-Eph signaling in embryonic tissue separation. Fagotto F, Winklbauer R, Rohani N. Cell Adh Migr. 2014;8(4):308-26. . Pubmed
2013
- Looking beyond the Wnt pathway for the deep nature of β-catenin. Fagotto F. EMBO Rep. 2013 May;14(5):422-33. Pubmed
- A molecular base for cell sorting at embryonic boundaries: contact inhibition of cadherin adhesion by ephrin/ Eph-dependent contractility. Fagotto F, Rohani N, Touret AS, Li R. Dev Cell. 2013 Oct 14;27(1):72-87. Pubmed
- EpCAM controls actomyosin contractility and cell adhesion by direct inhibition of PKC. Maghzal N, Kayali HA, Rohani N, Kajava AV, Fagotto F. Dev Cell. 2013 Nov 11;27(3):263-77. Pubmed
2012
- Cadherin-dependent differential cell adhesion in Xenopus causes cell sorting in vitro but not in the embryo. Ninomiya H, David R, Damm EW, Fagotto F, Niessen CM, Winklbauer R. J Cell Sci. 2012 Apr 15;125(Pt 8):1877-83. Pubmed
- Maternal Wnt/β-catenin signaling coactivates transcription through NF-κB binding sites during Xenopus axis formation. Armstrong NJ, Fagotto F, Prothmann C, Rupp RA. PLoS One. 2012;7(5):e36136. Pubmed
- Polyvalent DP1 keeps the Wnt pathway neat and tidy. Fagotto F. EMBO J. 2012 Aug 15;31(16):3377-9. Pubmed
- Proteomic analysis of differences in ectoderm and mesoderm membranes by DiGE. Wang R, Liu X, Küster-Schöck E, Fagotto F. J Proteome Res. 2012 Sep 7;11(9):4575-93. Pubmed
2011
- EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. Rohani N, Canty L, Luu O, Fagotto F, Winklbauer R. PLoS Biol. 2011 Mar;9(3):e1000597. Pubmed
- A method to separate nuclear, cytosolic, and membrane-associated signaling molecules in cultured cells. Liu X, Fagotto F. Sci Signal. 2011 Dec 13;4(203):pl2. Pubmed
2010
- The tumor-associated EpCAM regulates morphogenetic movements through intracellular signaling. Maghzal N, Vogt E, Reintsch W, Fraser JS, Fagotto F. J Cell Biol. 2010 Nov 1;191(3):645-59. Pubmed
2008
- Selective pharmacological targeting of a DEAD box RNA helicase. Lindqvist L, Oberer M, Reibarkh M, Cencic R, Bordeleau ME, Vogt E, Marintchev A, Tanaka J, Fagotto F, Altmann M, Wagner G, Pelletier J. PLoS One. 2008 Feb 13;3(2):e1583. Pubmed
- Plasma membrane recruitment of dephosphorylated beta-catenin upon activation of the Wnt pathway. Hendriksen J, Jansen M, Brown CM, van der Velde H, van Ham M, Galjart N, Offerhaus GJ, Fagotto F, Fornerod M. J Cell Sci. 2008 Jun 1;121(11):1793-802. Pubmed
- Inhibition of cell adhesion by xARVCF indicates a regulatory function at the plasma membrane. Reintsch WE, Mandato CA, McCrea PD, Fagotto F. Dev Dyn. 2008 Sep;237(9):2328-41. Pubmed
- Detection of nuclear beta-catenin in Xenopus embryos. Fagotto F, Brown CM. Methods Mol Biol. 2008;469:363-80. Pubmed
Cell adhesion and migration in embryonic development

François FAGOTTO
Chef d’équipe (Professeur)
Team Members
(Professeur) +33 (0)4 34 35 95 28 |
|
(IE-Recherche) +33 (0)4 34 35 95 25 |
|
(Stagiaire) +33 (0)4 34 35 95 28 |

Contact us
Replace the name and address below with that of the member to contact
firstname.name@crbm.cnrs.fr